Table of contents of the article
ToggleAlternaria black spot in cabbage is a fungal disease that affects cabbage leaves and affects its growth. This article from “WORLD OF PLANTS” provides tips for prevention and treatment
Identification of Alternaria black leaf spot in cabbage (Brassicas, Alternaria black leaf spot).
It is considered a common fungal disease in cabbage fields and represents an increasing threat to cabbage crops all over the world. It can infect all parts of the plant at all stages of growth (seedlings, leaves, petioles, stems, heads, flower discs, and seeds). The disease is caused by several types of Alternaria fungi and has a large number of plants. The host weakens the infected plants without causing their death.
Pathogen and conditions suitable for the development of Alternaria black spot disease in cabbage
There are several types of fungi:
Alternaria brassicae
Alternaria brassicicola
They affect: broccoli, Brussels sprouts, cabbage, cauliflower, Chinese cabbage, cabbage, and kale. And cruciferous wild plants.
Its ideal temperature is 18-24°C.
Alternaria raphani
It infects radishes and can infect other cruciferous plants.
Its ideal temperature is 20-30°C.
Relative humidity of 100-95% for 12-20 hours.
Free moisture on the plant is not less than 5 hours.
The fungus produces large quantities of spores, and the spores are spread throughout the fields by the wind, where they can be transmitted up to 1760 m by the wind. They are also transmitted to healthy plants through spraying water, agricultural equipment, workers, animals, and flea beetle insects when they feed on infected plants.
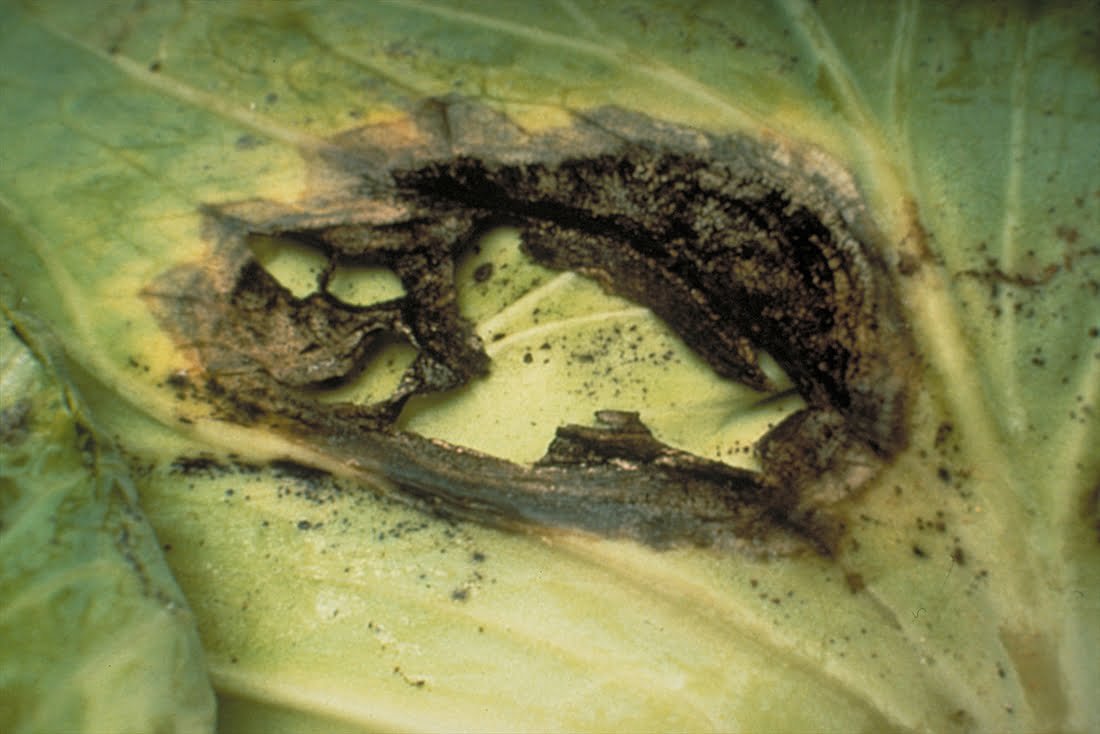
Symptoms of Alternaria black spot in cabbage
- It affects seedlings in the form of spots on the leaves and can cause their stunting and death.
- The leaves appear in the form of dark brown to black spots at any stage of growth and vary in size from a point to a diameter of 5 cm. They are clearer on the lower leaves of the plant. When the disease develops, the spots expand in diameter and take the shape of a bull’s eye and are in the form of circles. Concentric with a black center in the middle, it separates easily.
- It may cause yellowing of the affected leaves, and the infected spots may gather in large areas and the leaves may fall.
- Infection with the heads of cauliflower causes deterioration and damage to the entire head and loss of the marketing ability of the tablets. The injury appears in the form of black spots on the heads. These spots range from small pinpoints to larger circular spots with a diameter of up to several millimeters.
- As for cabbage, damage to the heads makes it impossible to store them.
- Infected heads and discs also become susceptible to injury withSoft mold Which we talked about in a previous article.
- Severe infestation may result in reduced photosynthesis, reduced plant activity, and decreased productivity.


Source of infection for Alternaria black spot in cabbage
1- Seeds, where the fungus can be present on the seed coat and remain viable for two years, or inside it in the form of mycelium and then remain viable for 12 years.
2- In the soil and infected plant waste.
The fungus was found to exist in two forms - microsclerotia and chlamydospores.
Prevention and treatment of Alternaria black spot in cabbage
1- Buy certified, disease-free seeds that can be treated with hot water.
2- The agricultural cycle for a period of not less than two years.
3- Deep cultivation of the soil.
4- Adherence to the specified planting distances and directing the planting rows with the direction of the wind in order for the plants to receive good air and sunlight with the specified planting density.
5- Getting rid of weeds.
6- Watering early in the morning and avoiding using overhead spraying.
7- Spraying with copper fungicides (copper chlorine oxide) 3 grams per liter of water. Spraying with mancozeb 3 grams per liter of water can be done when the disease appears and with an interval of 10-15 days between sprays, while getting rid of the affected leaves to improve air movement around the plants and reduce the number of spores present in the plant. big.
8- After harvesting, plant waste must be disposed of and the soil cultivated.
In conclusion, we would like to note that we, at the world of plants website, offer you all the necessary services in the world of plants, we provide all farmers and those interested in plants with three main services::-
- Artificial intelligence consulting service to help you identify diseases that affect plants and how to deal with them.
- Blog about plants, plant diseases and care of various crops ... You are currently browsing one of her articles right now.
- An application that provides agricultural consultations to clients, as well as a service for imaging diseases and knowing their treatment for free – Click to download the Android version from Google Play Store، Click to download the IOS version from the Apple App Store.
References:
- Bart P.H., Thomma J. 2003. Alternaria spp.: from general saprophyte to specific parasite. Molecular Plant Pathology 4(4): 225-236. [DOI:
- Bassey E.O., Gabrielson R.L. 1983. The effects of humidity, seed infection level, temperature and nutrient stress on cabbage seed
- Chevre A.M., Eber F., Brun H., Plessis J., Primard C., Renard M. 1991. Cyto- genetic studies of Brassica napus – Sinapsis alba hybrids from ovary culture and protoplast fusion. At- tempts to introduce Alternaria re- sistance into rapeseed. Proceedings of International Rapeseed Confer- ence 8: 346-351.
- Chirco E.M., Harman G.E. 1979. The effects of Alternaria brassicicola in- fection on Brassica seed vigor and viability. Journal of Seed Technolo- gy 3: 12-22.
- Conn K.L., Tewari J.P. 1986. Hypersensi- tive reaction induced by Alternaria brassicae in Eruca sativa, an oil- yielding crucifer. Canadian Journal of Plant Pathology 8: 348.
- 10.1016/0168-9452(88)90180-X] Degenhardt KJ, Petrie GA, Morrall
- R.A.A. 1982. Effects of temperature on spore germination and infection of rapeseed by Alternaria brassicae,
- Fahleson J., Rahlen L., Glimelius K. 1988. Analysis of plants regenerated from protoplast fusions between Brassica napus and Eruca sativa. Theoretical and Applied Genetics
- Hansen L.H. 1998. Intertribal somatic hybridization between rapid cycling Brassica oleracea (L.) and Came- lina sativa (L) Cranz. Euphytica
- Hansen LH, Earle ED 1995. Transfer of resistance to Xanthomonas cam-pestris pv. campestris (L.) by proto-plast fusion. Theoretical and Applied Genetics 91: 1293-1300. [DOI: 10.1007/BF00220944]
- Hansen LH, Earle ED 1997. Somatic hybrids between Brassica oleracea (L.) and Sinapis alba (L.) with re-sistance to Alternaria brassicae (Berk.) Sacc. Theoretical and Applied Genetics 94: 1078-1085. [DOI: 10.1007/s001220050518]




